British Medical College Pioneers New Treatment for Diabetes
Immunosuppressive Therapy “MonoPepT1De,” Prospective Panacea for Chronic Illness
May 10, 2022
Immunity – the physiological ability to distinguish between “self” and “non-self” and respond correspondingly – has enjoyed a good rap thus far. Balanced immunity, sustained by a balanced diet, exercise, and lifestyle, reinforces a protective response to infections and toxins. However, an overactive immune system may also trigger autoimmune responses, such as diabetes and allergies. In an autoimmune response, the immune system mistakenly starts attacking itself.
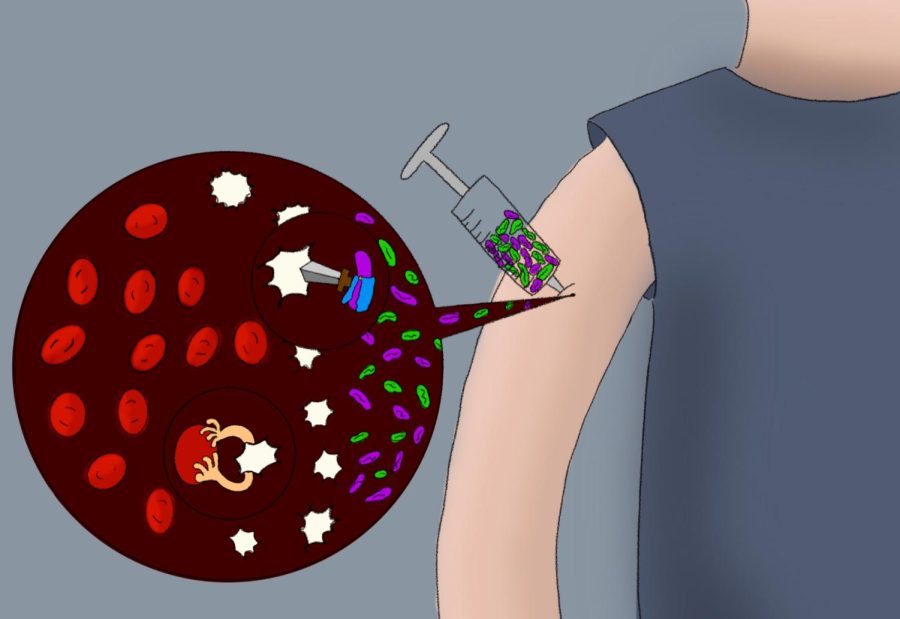
Type 1 diabetes mellitus (a.k.a. juvenile diabetes), dangerously prevalent in America and Europe, is defined as a “chronic condition in which the pancreas produces little or no insulin.” When we ingest carbohydrates, the pancreas secretes insulin in response to elevated blood sugar levels. That insulin then binds to the surface proteins of muscles and fat tissues that activate transport proteins that uptake glucose, thus being provided an energy source and maintaining a healthy range of blood sugar levels. In juvenile diabetes, however, the immune system, confused with bacteria, viruses, and toxin, mistakenly identifies pancreatic cells as “non-self,” producing autoantibodies that progressively destroy the pancreas, leaving a diabetic under total insulin deficiency. Then, what if immunity can be hampered to downregulate the autoimmune responses that cause diabetes?
Endocrinological researchers at King’s College London (KCL), led by Mohammad Alhadj Ali, are engineering an immunosuppressive therapy called “MonoPepT1De.” Purposefully injecting triggers of autoimmune responses in the muscle, Ali and his colleagues aimed to combat the overactivity of diabetic white blood cells simply by assigning them more work. This tricks the diabetic autoantibodies – having more substances (prediabetic antigen) to mark and destroy, the antibodies have less time to invest in destroying pancreatic cells.
Referring to 24 subjects that received the treatment over the course of a year, Ali published unprecedented results: compared to the placebo group, the treatment group all retained baseline insulin production, lower mean blood sugar level, and lower insulin therapy demand. Ali and his colleagues acknowledge in their research, “Our study extends experience with immunosuppressive therapy and provides further evidence that it has a very favorable safety profile, especially by comparison with biologic agents that carry the risk of acute toxicities.”
Even so, there are many obstacles. Ali’s team still fails to bring long-term effects, appropriate dosing, possible side effects, and individual variation into account. When these issues are addressed with long-term cohort study, this methodology – assigning the immune system more work instead of suppressing it – is a landmark establishing reverse-utilization for the treatment of lethal autoimmune disorders and redefining perspectives on the immune system.
Appendix
*Endocrinological researchers: researchers studying the regulation of blood chemistry by hormone glands
*Immunosuppressive therapy: therapy that functions by inhibiting immune system (often in autoimmune disorders)
*Prodiabetic antigen: biologic agent present in diabetic patients
*Baseline insulin production: normal rate of insulin production
*Mean blood sugar level: average blood sugar level over a span of period
*Safety profile: degree of safety in certain biomedical therapy
*Biologic agents: any bioactive molecule or microorganism that is used in a biomedical therapy
*Acute toxicity: characterized by quickly triggering lethal consequences
*Cohort study: a study of a group with certain characteristics over a period of time
Reference (APA-cited)
Alhadj Ali M, Liu YF, Arif S, Tatovic D, Shariff H, Gibson VB, Yusuf N, Baptista R, Eichmann M, Petrov N, Heck S, Yang JHM, Tree TIM, Pujol-Autonell I, Yeo L, Baumard LR, Stenson R, Howell A, Clark A, Boult Z, Powrie J, Adams L, Wong FS, Luzio S, Dunseath G, Green K, O’Keefe A, Bayly G, Thorogood N, Andrews R, Leech N, Joseph F, Nair S, Seal S, Cheung H, Beam C, Hills R, Peakman M, Dayan CM. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci Transl Med. 2017 Aug 9;9(402):eaaf7779. doi: 10.1126/scitranslmed.aaf7779. PMID: 28794283.
DiMeglio, L. A., Evans-Molina, C., & Oram, R. A. (2018). Type 1 diabetes. Lancet (London, England), 391(10138), 2449–2462. https://doi.org/10.1016/S0140-6736(18)31320-5.
Young, S. Y. (2019). Rotavirus vaccines may lower kids’ chances of getting type 1 diabetes. Washington, DC: Science News. https://www.sciencenews.org/article/rotavirus-vaccine-kids-type-1-diabetes.































![[Podcast] Bet on Bonnie Episode 2: Sophie Lee](https://jetsflyover.com/wp-content/uploads/2024/05/jbspodcasts-1200x951.png)

![[Podcast] Jets Jukebox Episode 1: Bleachers, Self-Titled](https://jetsflyover.com/wp-content/uploads/2024/04/Jets-Jukebox-LOGO-1-1200x1200.png)
![[Podcast] Eco-Lution Episode 3: Freshmen explore alternatives to paper straws](https://jetsflyover.com/wp-content/uploads/2024/03/Ecolution_Podcast_Logo-1200x1200.png)